SMOOTHER, TIGHTER, CONTOURED SKIN FOR ALL.
Solta Medical supports the universal quest to feel better about your appearance no matter what your age, shape, size or color. We’ve intentionally assembled a portfolio of highly useful devices that can treat all types of patients for a wide variety of the most common skin concerns.
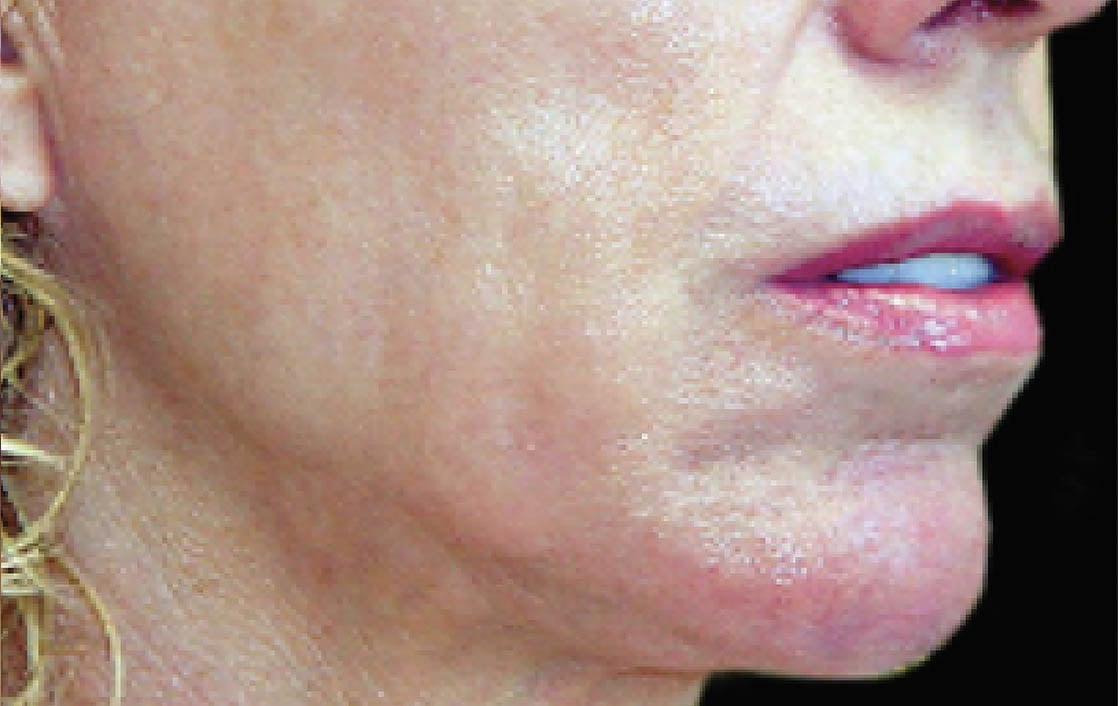
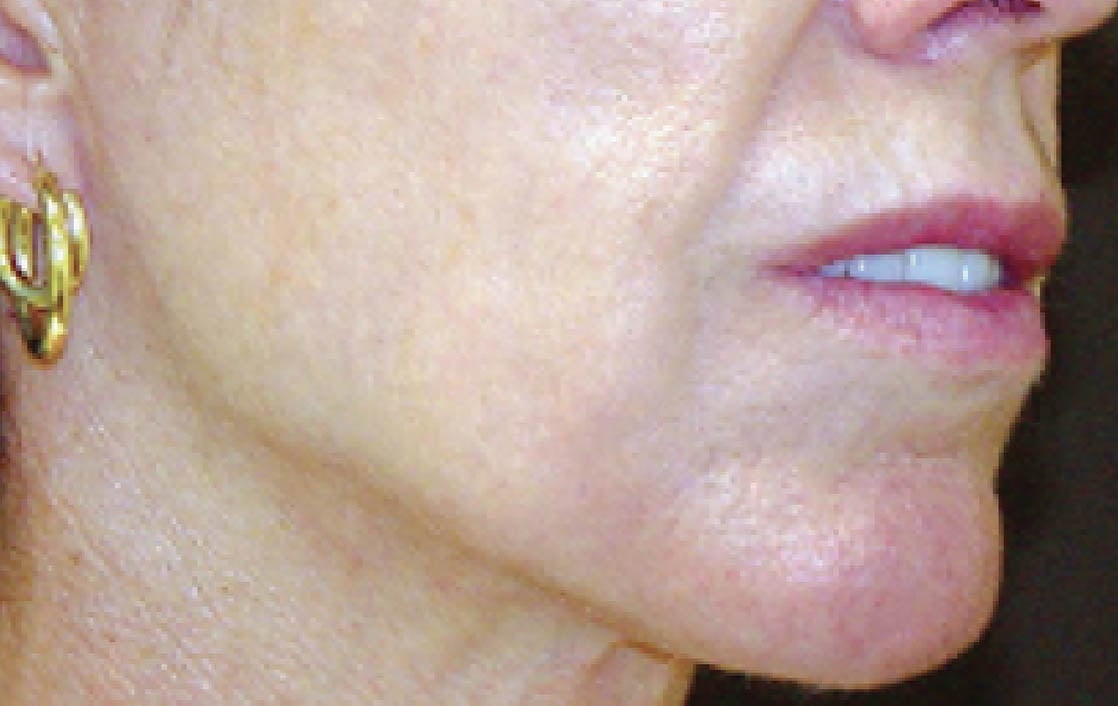
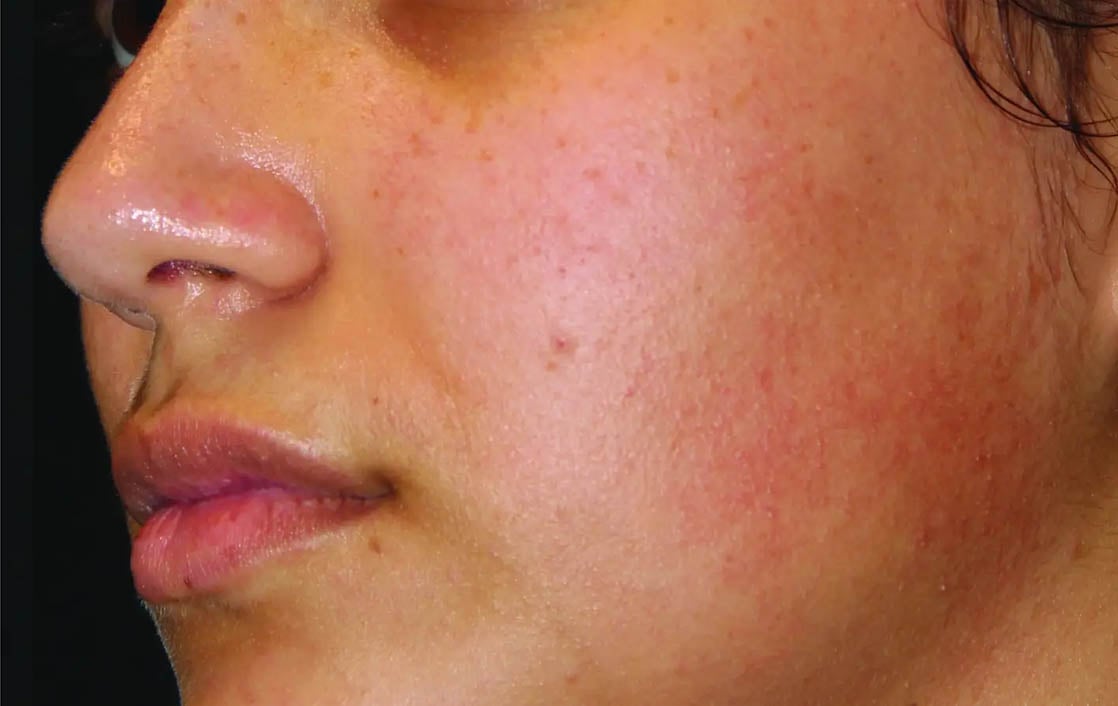
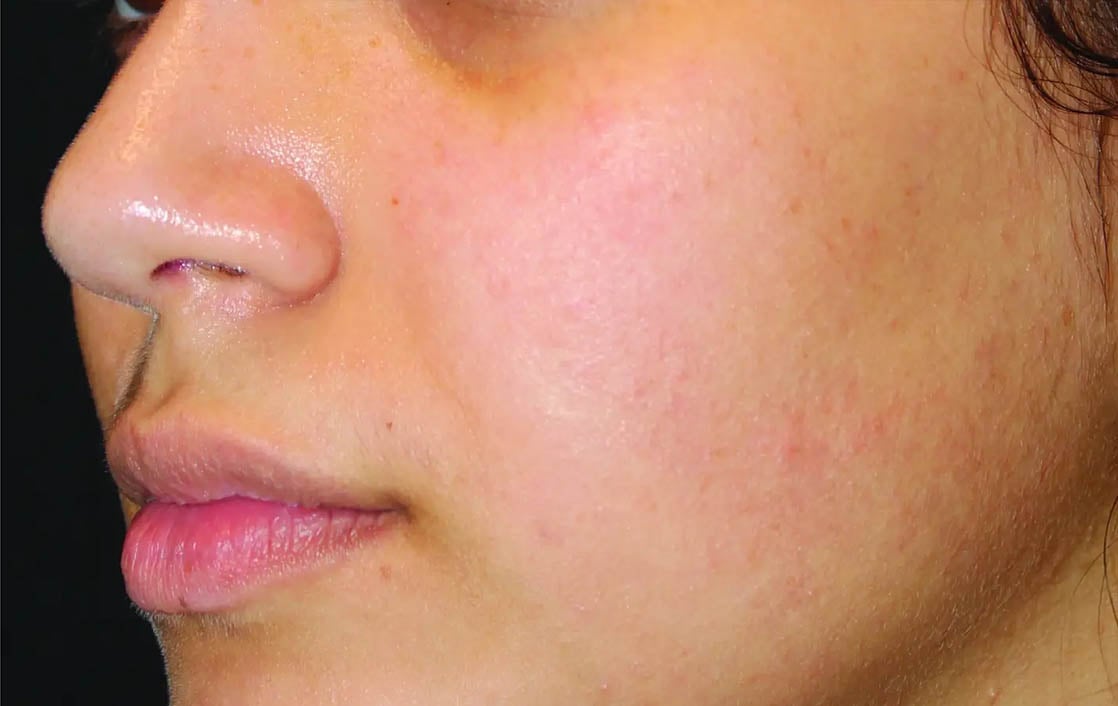
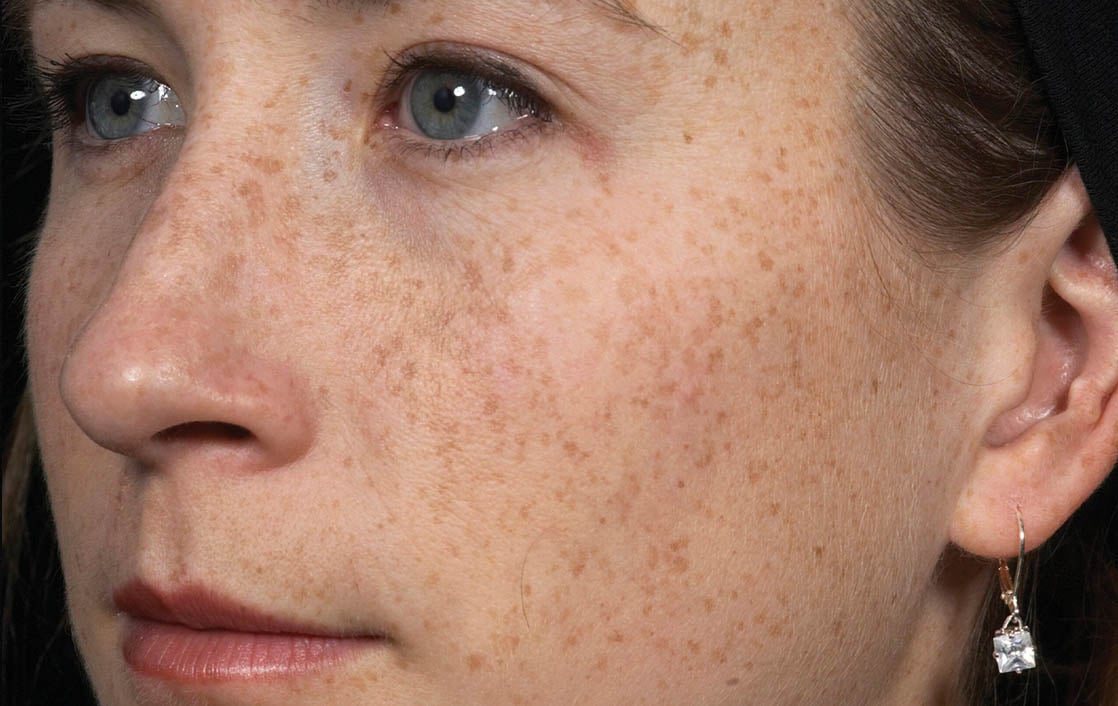



Unretouched photos. Individual results may vary.
Before and after photos may be a compilation of results from all generations of Thermage devices including NXT, CPT, and FLX.
RESULTS THAT MAKE A DIFFERENCE.
We’re proud of the impact our treatments have had on millions of lives over the past 20+ years. Our longevity also speaks to the consistent partnership and support that we provide to our customers who have been with us through the process.
SOLTA STORIES
Our practice partners discuss their experience with Solta.
MAKE AN IMPACT WITH SOLTA.
Join the thousands of practices that offer their patients Solta devices.
Contact a Representative